Access Denied
IMPORTANT! If you’re a store owner, please make sure you have Customer accounts enabled in your Store Admin, as you have customer based locks set up with EasyLockdown app. Enable Customer Accounts
Glucosamine Intensive Care
Vendor: Metagenics
Product Details
|
To access our exclusive Practitioner range supplements, you have the following options:
Looking for Personalized Health Guidance?
|
-
Joint Support Complex To Assist Mobility.
Ingredients in Glucosamine Intensive Care have been shown to or may:
- Protect joint cartilage in mild osteoarthritis.
- Support knee joint mobility and range of movement in athletes.
- Convenient, twice daily tablet for improved compliance.
Clinical Benefits:
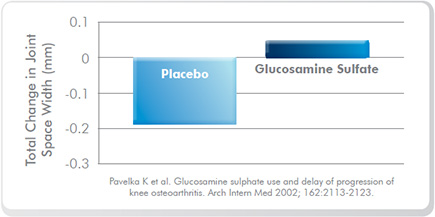
- Several randomised, placebo-controlled, double-blind trials have shown 1500 mg of glucosamine sulfate as assisting in the management of mild osteoarthritis: In one study, subjects taking the placebo experienced progressive joint space narrowing due to cartilage damage, whereas subjects taking glucosamine did not demonstrate joint space narrowing (Figure One). A 2005 meta-analysis of studies in mild osteoarthritis found 1500 mg of glucosamine daily was superior to placebo in terms of pain and functional impairment in 20 randomised clinical trials. The suggested mechanism of action has been demonstrated in an animal study where glucosamine inhibited IL-1ß, reducing inflammation and swelling. Glucosamine is required for the production of proteoglycans, mucopolysaccharides and hyaluronic acid, the substances that make up joint tissue.
- May improve joint mobility and range of movement: Glucosamine has been shown, in a randomised, placebo-controlled double-blind trial, to support joint mobility for sports knee injuries in athletes. Athletes with a recent knee injury were given 1500 mg glucosamine per day and after 28 days there was significant improvement in knee flexion and extension in those taking glucosamine compared with placebo. This may be due to its function in reducing collagen breakdown associated with exercise.